In This Issue
Message From the
Executive Director
Advocacy Update!
Your Investment at Work
 Now Visit VSI Now Visit VSI
on Facebook
 And Twitter And Twitter
Breaking Down Insurance Barriers!
PLUS- Persuasive Sample Letters
What's On Your Mind?
- Is it OK to apply topical
ointments around the eyes?
- Is it safe to remove goggles
during light treatments?
- Can you treat vitiligo with
dandruff shampoo?
Medical News Updates
- Clinuvel-Scenesse Update!
- Target Identified for
Potential Vitiligo Treatment
- Are Vitiligo Patients
More Susceptible to
Autoimmune Disorders?
Research & Clinical Trials
Bibliography
Earn funding for VSI
When you Shop!
View Past Newsletters
- Winter Edition 2013
- Fall Edition 2013
- Summer Edition 2013
- Spring Edition 2013
- Winter Edition 2012
- Fall Edition 2012
- Summer Edition 2012
- Spring Edition 2012
- Winter Edition 2011
- Fall Edition 2011
- Spring Edition 2011
- Winter Edition 2010
- Fall Edition 2010
VSI Medical and Scientific
Advisory Committee
Pearl E. Grimes, M.D., Committee Chair
Ted A. Grossbart, Ph.D.
Sancy A. Leachman, M.D.
I. Caroline Le Poole Ph.D.
Mauro Picardo, M.D.
Nanette B. Silverberg, M.D.
Richard A. Spritz, M.D.
Alain Taieb, M.D., Ph.D.
Wiete Westerhof, MD, Ph.D.
For more information
on VSI's MSAC Click Here |
| |
| |
How to Log In:
Have you have forgotten your Login ID and/or Password?
No Problem!
Just go to the
Community Page
Scroll down to
the login box.
LOOK UNDERNEATH!
You'll see instructions to have them sent to the email address on your account. |
|
How to Log In:
Have you have forgotten your Login ID and/or Password?
No Problem!
Just go to the
Community Page
Scroll down to
the login box.
LOOK UNDERNEATH!
You'll see instructions to have them sent to the email address on your account.
|
|
| |
How to Log In:
Have you have forgotten your Login ID and/or Password?
No Problem!
Just go to the
Community Page
Scroll down to
the login box.
LOOK UNDERNEATH!
You'll see instructions to have them sent to the email address on your account.
|
|
| |
| Contact Us
Online
VitiligoSupport.org
Email Contact Us
Postal Mail Address
Vitiligo Support International
P.O. Box 3565
Lynchburg Va 24503
Phone
(434) 326-5380 |
|
Message From the Executive Director

Dear Members and Friends of VSI,
We have all experienced the tremendous frustration, or at least heard stories from others, about hitting a brick wall with insurance reimbursement for vitiligo treatments. Having vitiligo already places a high burden on patients, and adding the barriers patients face in obtaining reimbursement increases that burden greatly. Know that you are not alone! Vitiligo Support International is on the front lines, gauging the problems vitiligo patients face with reimbursement and offering you the tools to guide you through the maze of insurance reimbursement and increase your chances of success when requesting reimbursement and/or appealing denials for a claim.
VSI sent out a survey to its membership last fall to better understand the difficulties faced by vitiligo patients with insurance reimbursement, and we learned about some interesting trends. (See survey results below.) We also have been gathering information from many of you about your difficulties and your successes. This way, we can use your experiences to help others and better understand the insurance landscape so we can begin to strategize about how to change it.
Wouldn’t it be great to have all of the information you need for handling insurance claims at your fingertips? We wanted to offer you answers to your many questions that come in to our office and through our message boards, and provide you with top resources on insurance reimbursement. Even better, we wanted to make that information easily accessible by placing all the information in one location, that you need to make your quest for reimbursement. We have included tips on insurance and sample letters for appealing a denial.
VSI can provide you with the information you need, but YOU provide the key to your success. Be an empowered patient! Educate yourself, and be proactive. If you take charge of your care, your chances for success are increased significantly. VSI is here to help you do just that. We’re committed to increasing your access to insurance coverage and have provided the tools you need to get started.
In addition to our lead topic on health insurance, we know that clinical trials and medical news updates are always important to you. So what’s in the pipeline for vitiligo? As always, find out the latest information in this newsletter!
Sincerely,
Jackie Gardner
Executive Director

Advocacy Update
Your Investment at Work!

VSI Director Jackie Gardner recently attended the 2014 American Academy of Dermatology Annual Meeting in Denver, Colorado. In addition to attending the vitiligo medical education sessions, she addressed the Vitiligo Working Group (VWG), an international panel of esteemed researchers and clinicians committed to improving current treatments, increasing research efforts, and perhaps most importantly, gaining a better understanding of what patients want and need.
  The focus of Ms. Gardner’s presentation centered on the many challenges faced by vitiligo patients, including such topics as the lack of understanding by many in the medical field of the emotional impact of the disease; the inequality of access to treatment; the burden of the expense of treatments, coupled with problems presented by denial of insurance coverage; and the need for additional financial support of VSI to enable better representation of the needs of the vitiligo community. The focus of Ms. Gardner’s presentation centered on the many challenges faced by vitiligo patients, including such topics as the lack of understanding by many in the medical field of the emotional impact of the disease; the inequality of access to treatment; the burden of the expense of treatments, coupled with problems presented by denial of insurance coverage; and the need for additional financial support of VSI to enable better representation of the needs of the vitiligo community.
 
While attending the VWG meeting, Ms. Gardner had the opportunity to hear an update on the clinical trial for Scenesse, developed by Clinuvel.
After the meeting, she took a moment to speak with Clinuvel’s CEO and Director, Philippe Wolgen.
VSI needs your financial support in order to
maintain our work at this level.
Show Your Commitment Today!
Invest in Your Future - Support VSI
Become a Supporting Member: Click Here
Make a General Donation Through VSI: Click Here
Make a General Donation with PayPal

|

|
This organization is a Silver-level GuideStar Exchange
participant, demonstrating its commitment to transparency. |

Tackling Health Insurance
and Reimbursement for Vitiligo Treatments
  Obtaining insurance coverage for the most basic and standard medical treatments for vitiligo can present a major challenge. The insurance industry often labels such treatments as being merely “cosmetic” rather than medically necessary, and they use this description to deny claims. Vitiligo patients face a much more difficult battle than those with similar diseases, because standard treatments for vitiligo are not FDA-approved for vitiligo. In comparison, those same treatments might be approved for psoriasis, psoriatic arthritis or alopecia areata. Ideally, companies would engage in randomized, placebo-controlled clinical trials for these treatments using vitiligo patients and request FDA-approval for those that demonstrate positive outcomes. Unfortunately, once a therapy is approved for a similar disease, companies have little incentive to spend the millions of dollars it would take to enter trials for vitiligo. This often leaves the vitiligo patient scrambling for insurance reimbursement for treatments that are considered off-label. Obtaining insurance coverage for the most basic and standard medical treatments for vitiligo can present a major challenge. The insurance industry often labels such treatments as being merely “cosmetic” rather than medically necessary, and they use this description to deny claims. Vitiligo patients face a much more difficult battle than those with similar diseases, because standard treatments for vitiligo are not FDA-approved for vitiligo. In comparison, those same treatments might be approved for psoriasis, psoriatic arthritis or alopecia areata. Ideally, companies would engage in randomized, placebo-controlled clinical trials for these treatments using vitiligo patients and request FDA-approval for those that demonstrate positive outcomes. Unfortunately, once a therapy is approved for a similar disease, companies have little incentive to spend the millions of dollars it would take to enter trials for vitiligo. This often leaves the vitiligo patient scrambling for insurance reimbursement for treatments that are considered off-label.
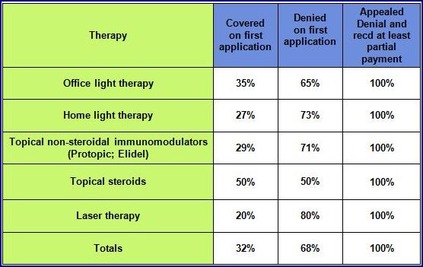
Denials for vitiligo patients most often are attributed to the insurance company designating a therapy as cosmetic, not medically necessary, and/or being experimental or investigational in vitiligo and prescribed as off-label use. Statistically, about 40% of appeals are successful, so VSI urges you to always appeal a denial.
2013 VSI Survey on Insurance Reimbursement
VSI surveyed vitiligo patients in the fall of 2013 about health insurance and reimbursement rates. Respondents cited a wide range of affiliation with specific insurance companies, and the insurance company did not appear to affect the outcome of coverage. Overall, the responses VSI received reveal some interesting trends:
- Astoundingly, all of those who appealed insurance denials reported receiving at least partial reimbursement.
- On average, 68% of those who applied for insurance for any vitiligo-related treatment were denied reimbursement on the first application.
- More than twice as many applicants were denied coverage than accepted for both home light therapy and topical non-steroidal immunomodulators such as Protopic® or Elidel®.
- Only 1 out of 5 applications for laser therapy reimbursement was accepted on the first try. More people were denied coverage for laser therapy than any other therapy (80% for initial applications).
- Topical steroids as well as light therapy provided in the office setting were the treatments most likely to be covered by health insurance.
Table Showing Results of 2013
VSI Survey on Insurance Reimbursement
Tips on Applying for Insurance Reimbursement
- Know your insurance policy and what it covers.
- Diagnosis is billed using the International Classification of Diseases (ICD) coding system. The diagnostic code for vitiligo is 709.01.
- Investigate how your policy handles a specific treatment. For example, for a home light unit, the policy needs to include Durable Medical Equipment (DME) coverage.
- Note whether prior authorization is needed for a therapy.
- Understand any co-pays and how much you will be expected to contribute to the cost of a therapy.
- Know whether your insurance company requires “step therapy,” which means you must try and fail one therapy before the next level of therapy can be covered.
- Do your homework before submitting a claim.
 Some treatments require pre-authorization.
Be aware whether this is the case with your therapy. Some treatments require pre-authorization.
Be aware whether this is the case with your therapy.
- Many light companies recommend that you let them file the claim.
- Make sure your medical records are accurate.
- Maintain copies of your medical records. You have the right to receive copies of all of your medical records. Note that you can be charged a copy fee.
- Include a Letter of Medical Necessity.
- See sample “Letter of Medical Necessity for Home Light Therapy” below. Sample language for alternate therapies for vitiligo patients can be taken from the sample appeal letters below.
- Always appeal denials!
- Appeal a denial at every level. Most vitiligo patients receive at least partial reimbursement upon appealing a negative decision from their insurance company.
- Be persistent! Don’t give up!
- Involve your doctor in helping you respond to a denial.
- Documentation is crucial! In addition to making sure you have the necessary documentation showing that your case meets the insurance provider’s guidelines and demonstrates medical need, maintain records of your communication with the insurance company. Document every time you speak or hear from a company representative; Record the person’s name, date, time and key messages from the conversation.
- Familiarize yourself with your insurance company’s guidelines and deadlines for appeal. This information is usually included in the denial letter.
- Understand why you were denied, so you can address the insurance company’s reason(s) directly.
- If you are communicating with the Customer Service office of the insurance company and are dissatisfied with the response, ask for a Nurse Case Manager or a Supervisor who might be more understanding of your situation.
- If you did not previously include a Letter of Medical Necessity, make sure you include one for the appeals process.
- When possible, demonstrate that treatment is more cost-effective than alternatives or non-treatment. See sample language in the information provided on the Letter of Medical Necessity.
- If you are still denied following the final round of appeals for a prescription treatment, contact the advocacy or patient assistance program for the company that produces the treatment. Most companies have divisions that take applications for financial assistance for their therapies.
- If you are unsuccessful with the appeal process, consider contacting your state insurance commission. Information can be found at www.kff.org/consumerguide/7359.cfm.
  
How to Draft an Appeal Letter
Include the following information and documentation:
- Your policy and claim numbers, employer name if your policy is through an employer, and the full name of the insured
- The therapy or procedure for which you were denied and why the denial letter stated you were denied
- Medical records that back up your diagnosis and medical problem that relates to the therapy in question
- A cost-benefit analysis when relevant - For example, you can compare the cost savings of purchasing home light therapy equipment compared to the higher cost of receiving light therapy in an office setting. If your office light therapy costs, for example, about $50 per treatment, the annual cost at three times a week would be $7,500. Compare the $7,500 cost for office light therapy to the home equipment cost of about $2,200, and you have demonstrated a savings to the insurance company of more than $5,000.
- Letter of Medical Necessity:
Click Here to View Sample Letter of Medical Necessity for Home Light Therapy
- If the Letter of Medical Necessity is not signed by your physician, have your physician provide a letter of support that includes the reason for recommending or prescribing your therapy.
- Quotes from your health insurance policy that are helpful to your case – For example, one VSI member stated that her policy cited that coverage was provided for laser treatment in psoriasis patients because the treatment is coded for inflammatory skin disease. By quoting this back to the insurance company and providing documentation that vitiligo involves a similar inflammatory response, the chance for success of an appeal is much greater.
- Two or more articles from respected medical journals backing your claim of medical necessity
- Refer to the VSI website as an authoritative source of medical information on vitiligo.
Response to denial designation that a treatment is “cosmetic”:
 
- Breast reconstructive surgery is also cosmetic, but usually covered. We recognize the importance of how loss of a breast affects a person’s psychological and social well-being. This is true of the depigmentation that occurs in vitiligo as well.
- Wigs for those with alopecia areata require prior authorization as DME but are then covered as a legislatively mandated benefit. Wigs would fall under the “cosmetic” category, but our government recognizes the devastating mental and social consequences of dealing with hair loss. This is true of the depigmentation that occurs in vitiligo as well.
- Disease-modifying treatments such as photochemotherapy – psoralen plus UVA therapy or PUVA – are covered for alopecia areata and that and other light therapies are covered for psoriasis and psoriatic arthritis when provided by a physician in a clinic or other outpatient setting. While co-pays might be high, broad acceptance exists for these treatments as medically necessary in closely related diseases. Light therapy is a disease-modifying treatment that is scientifically proven effective, widely accepted in the medical community for treating and managing vitiligo, and access to coverage should not be any different for vitiligo than for psoriasis and alopecia areata.
Click Below for Individual Sample Appeal Letters
- Home Light Therapy
- Office Light Therapy
- Laser Treatment
- Topical Immunomodulatory Ointment (Protopic, Elidel)
Affordable Care Act (ACA):
Under this act, insurance companies cannot turn you down for coverage because of a pre-existing condition such as vitiligo. Health plans also have to cover what is known as “essential health benefits,” which include chronic disease management and prescription drugs. In addition, the law also ends lifetime limits on coverage. This national healthcare reform law was enacted in March 2010. The first open enrollment ended March 31, 2014, and the next period of enrollment is slated for November 15, 2014 – January 15, 2015. For more information on the ACA, visit:
http://www.hhs.gov/healthcare/rights/
http://www.nationalhealthcouncil.org/ |
(Back to Top)


What's On Your Mind?
Q. Is it OK to put the topical ointments around the eyes?
- If you are trying to treat depigmented areas around the eyes, you need to be careful not to get the ointment or cream too close to the eye where it could get warm and possibly leach into the eye. If you are worried, you might ask your doctor about sparingly applying the ointment or cream just below the eyebrow. Many VSI members have reported that after blinking a few times, it seems to spread onto the lower part of the eyelid without getting into the eye.
Q. I am using NB-UVB light, but wearing goggles. Is it safe to remove the goggles to expose my eyelids to the light?
  Research has shown that NB-UVB light does not penetrate the eyelid. As long as no psoralen is being used (as with PUVA), the patient is old enough to understand they must keep their eyes closed at all times, and has an annual eye exam, it should be safe to expose the eyelid to NB-UVB light. Research has shown that NB-UVB light does not penetrate the eyelid. As long as no psoralen is being used (as with PUVA), the patient is old enough to understand they must keep their eyes closed at all times, and has an annual eye exam, it should be safe to expose the eyelid to NB-UVB light.
Source: Amit G. Pandya, MD, Department of Dermatology, University of Texas Southwestern Medical Center, Dallas, Texas)
Q. Is it true that you can treat vitiligo with dandruff shampoo?
  Unfortunately, this is not true. There are various skin conditions that can cause “hypo-pigmentation,” or loss of pigment. One of the more common, known as tinea versicolor, is caused by a yeast-type fungus, and as such, is treated with antifungal medications. Because (anti) dandruff shampoos contain antifungal ingredients, many times this type of “over-the-counter” product will clear up a condition like tinea versicolor. Vitiligo is not a skin fungus, and cannot be treated with antifungal medications. Unfortunately, this is not true. There are various skin conditions that can cause “hypo-pigmentation,” or loss of pigment. One of the more common, known as tinea versicolor, is caused by a yeast-type fungus, and as such, is treated with antifungal medications. Because (anti) dandruff shampoos contain antifungal ingredients, many times this type of “over-the-counter” product will clear up a condition like tinea versicolor. Vitiligo is not a skin fungus, and cannot be treated with antifungal medications.
Read more about vitiligo treatments on VSI’s Treatment page

Medical News Updates
Scenesse Found Beneficial for Darker Complexions
Phase 2 to be held in Singapore
  Vitiligo patients with darker skin complexions demonstrated the most pronounced benefit in Phase I trials for Scenesse combined with NB-UVB light therapy. This announcement marks a potential breakthrough for vitiligo patients with darker complexions and is especially exciting because of the hope it brings to those with darker complexions and who live in countries in which the negative social stigma of vitiligo is especially devastating. As a result of the Phase 1 results, Phase 2 for the use of Scenesse combined with NB-UVB light therapy will be launched this year in Singapore to more closely observe the results of those with a darker complexion. Vitiligo patients with darker skin complexions demonstrated the most pronounced benefit in Phase I trials for Scenesse combined with NB-UVB light therapy. This announcement marks a potential breakthrough for vitiligo patients with darker complexions and is especially exciting because of the hope it brings to those with darker complexions and who live in countries in which the negative social stigma of vitiligo is especially devastating. As a result of the Phase 1 results, Phase 2 for the use of Scenesse combined with NB-UVB light therapy will be launched this year in Singapore to more closely observe the results of those with a darker complexion.
Clinuvel Pharmaceuticals Limited announced the launch of the CUV103 trial this winter. The CUV103 trial tests the use of afamelanotide (Scenesse), a repigmentation therapy. The latest trials hope to confirm earlier results showing that a combination of Scenesse and NB-UVB light therapy provides repigmentation at a greater and faster rate than NB-UVB light therapy alone.
The randomized placebo-controlled clinical trial (the gold standard in clinical trials) plans to enroll about 60 patients over a three-month period, and the trial itself is expected to run for seven months with patients followed for an additional 3 months. As in earlier trials, patients in the Phase II trial will receive narrowband ultraviolet B (NB-UVB) light therapy twice weekly in addition to the new therapy, Scenesse. The study will be carried out at the National Skin Centre in Singapore. Assessment will include the use of two prevalent scoring systems for vitiligo and vitiligo repigmentation - the Vitiligo Area Scoring Index or VASI and the Vitiligo European Task Force or VETF, the length of time it takes for repigmentation to occur, digital photography, questionnaires on quality of life, and investigator statements and patient surveys.
The new trial builds on initial CUV102 trials for which results were released in December 2012 and follow-up results published in July 2013. Primary and secondary objectives were met in the initial trial and focused primarily on the extent of repigmentation and, secondly, on the time it took to repigment. No significant safety issues were reported.
Afamelanotide (Scenesse) activates eumelanin in the skin, which is the dark pigment known to provide protective properties against light and UV radiation. The drug is delivered via a dissolvable implant injected underneath the skin. Study design was executed in the U.S. by Henry W. Lim, MD, Pearl Grimes, MD (VSI Medical Board Chair), and Mark G. Lebwohl, MD (President Elect of the American Academy of Dermatology), and in Singapore by Steven Thng, MD. Singapore announced last fall that the Ministry of Health was committing about $100 million for skin research.
VSI has been monitoring this new therapy and keeping you informed about progress via the VSI website and past newsletters. Readers can review the Fall 2013, Summer 2013 and Winter 2012 issues for past reports on Scenesse. Watch for the latest information on clinical trial recruitment in future VSI newsletters.
Potential Target for Vitiligo Treatment Identified
  A new potential target for vitiligo treatment has been identified by a team of researchers led by John E. Harris, MD, PhD at the University of Massachusetts Medical School in Worcester, Massachusetts. The target involves a chemokine, which is a protein that is active in the communication between cells and is involved in immune function. The chemokine, called CXCL10, is elevated in the skin and blood of vitiligo patients and has been found to play a critical role in the progression and maintenance of depigmentation in a mouse model of vitiligo. In their study, Harris and colleagues found that mice lacking CXCL10 or that were treated with an antibody neutralizing their CXCL10 had minimal depigmentation. This discovery points to CXCL as a potential target for developing an effective therapy for vitiligo. A new potential target for vitiligo treatment has been identified by a team of researchers led by John E. Harris, MD, PhD at the University of Massachusetts Medical School in Worcester, Massachusetts. The target involves a chemokine, which is a protein that is active in the communication between cells and is involved in immune function. The chemokine, called CXCL10, is elevated in the skin and blood of vitiligo patients and has been found to play a critical role in the progression and maintenance of depigmentation in a mouse model of vitiligo. In their study, Harris and colleagues found that mice lacking CXCL10 or that were treated with an antibody neutralizing their CXCL10 had minimal depigmentation. This discovery points to CXCL as a potential target for developing an effective therapy for vitiligo.
Are Vitiligo Patients More Susceptible
to Developing Additional Autoimmune Disorders?
VSI previously reported on other autoimmune conditions that tend to occur in vitiligo patients in its Summer 2011 newsletter. Now, two studies just out concur with previous investigations showing that vitiligo patients can have additional autoimmune disorders.
The first study, an Italian study published in March 2014, looked at 175 vitiligo patients and found that autoimmune thyroid disease was the most prevalent autoimmune disorder that occurred in vitiligo patients, with 37% of those with vitiligo also having autoimmune thyroiditis. Nearly 42% of all subjects those who underwent laboratory tests had at least one type of autoantibody, or antibody directed against one’s own tissues and help a physician diagnose a specific autoimmune disease. The most common autoantibodies found were two that are involved in thyroid disease, anti-thyroperoxidase (25.6%) and anti-thyroglobulin (23.4%). The next most common were antinuclear antibodies (ANA), which were found in 16.8% of patients and can indicate the existence of other autoimmune diseases such as rheumatoid arthritis or lupus. A smaller percentage of patients (nearly 8%) were found to have anti-gastric parietal cell antibodies which target the stomach and lead to difficulty absorbing B12 and can cause pernicious anemia.
The second study, an eleven year retrospective review of the vitiligo patient population at Henry Ford Health Systems (HFHS) in Detroit MI, was presented at the American Academy of Dermatology conference in March 2014 and examined 1,098 vitiligo patients in the U.S. Similar to the other study, the most common autoantibodies targeted the thyroid, with 12.3% being diagnosed with thyroid disease, 27.1% testing positive for anti-thyroid peroxidase (TPO) and 10.4% testing positive for anti-thyroglobulin (Tg). Antinuclear antibodies (ANA) were found in 18.5% of the vitiligo patients, which is close to the rate of the earlier study. Among those who were tested, autoimmune diseases diseases found with greater frequency were, in order of prevalence, autoimmune thyroid, alopecia areata, inflammatory bowel disease, type 1 diabetes mellitus, pernicious anemia, systemic lupus erythematosus, myasthenia gravis and multiple sclerosis.
Conclusions from both studies indicate that vitiligo patients and their physicians should be on the watch for signs and symptoms of other autoimmune diseases, especially thyroid disease.
Back to Top
Research & Clinical Trials
Clinical Trial In Arizona for Novel Topical Treatment
Photocil (Topical) for the Treatment of Vitiligo
Principal Investigator: Sharon Keene, MD;
Study Location: Physicians Institute, Tucson, Arizona
Photocil is a topical cream that works with natural sunlight instead of using traditional phototherapy equipment. It allows only those wavelengths of Ultraviolet B (UVB) light that are most beneficial for vitiligo patients through while protecting users from non-therapeutic radiation.
Study Goal: The study aims to assess the safety and effectiveness of Photocil in the treatment of vitiligo.
Eligibility Requirements:
- Must be 18 – 65 years of age
- Diagnosed with vitiligo and diagnosis must be confirmed by a dermatologist
- Must have vitiligo lesions affecting a minimum 5% of the facial, leg or arm surface areas
Exclusion Criteria – Patients who:
- Did not respond to prior phototherapy treatment
- Completed phototherapy for same lesion(s) in last 6 months
- Has previous history of skin cancer
- Has previous history of photosensitivity
- Has a history of herpes (HSV I or II) outbreaks
- Has previous history of autoimmune disease may be excluded at investigator's discretion
- Is currently taking of immunosuppressive or photosensitizing drugs
- Plan to use antibiotics, anti-fungal, calcineurin inhibitors or other drugs that may cause photosensitivity during the study period. These patients may be excluded at investigator's discretion.
- Is pregnant or lactating
Explanation:
NB-UVB light therapy is commonly used to treat vitiligo, but access to treatment and compliance can be drawbacks. Light therapy can involve trips to a physician’s office, and insurance reimbursement sometimes can prove difficult (see lead article on insurance). A randomized placebo-controlled trial, this pilot study will use the Vitiligo Area Severity Index (VASI) to measure the outcome. If positive results are obtained, a larger study will take place. The study sponsor is Applied Biology, Inc.
For more information - or to sign up: Call: 520-290-5555 |
Melanocyte-Keratinocyte Transplant Procedure
Opportunity in Detroit!
Comparative Study Using Dermabrasion versus CO2 Laser
and Collagen Dressing versus Vaseline Gauze in MKTP
Principle Investigator: Iltefat Hamzavi, MD
Study Location:
Henry Ford Department of Dermatology,
3031 West Grand Boulevard Detroit, MI 48202
The melanocyte keratinocyte transplant procedure (MKTP) involves transplantation
of the skin cells that produce pigment from your normal skin to the depigmented skin. The procedure takes approximately 4 hours and is done under local anesthesia.
This is a prospective, open-label, parallel study comparing two different techniques
for preparing the depigmented skin (carbon dioxide laser versus dermabrasion)
and comparing two different wound dressings (collagen dressing versus vaseline impregnated gauze) for the melanocyte keratinocyte transplant procedure.
Eligibility Requirements:
- Must be 18 years of age or older
- Must have depigmented patches of skin
Exclusion Criteria:
- History of acral vitiligo (vitiligo on the hands or feet)
- Unstable vitiligo, defined as any new or enlarging areas of
depigmentation within the last 6 months)
- History of thickened scars or keloids
- History of koebnerization (getting new areas of
depigmentation at sites of trauma, such as a cut, scrape, or burn)
The vitiligo patch will be divided into four quadrants. Each quadrant will be
treated with the melanocyte keratinocyte transplant procedure, as follows:
- CO2 laser for denuding the epithelium Collagen dressing
- CO2 laser for denuding the epithelium Vaseline impregnated gauze dressing
- Dermabrasion for denuding the epithelium Collagen Dressing
- Dermabrasion for denuding the epithelium Vaseline impregnated gauze dressing
The patient will return to the clinic for the dressing removal 1 week post-procedure. Repigmentation of the treated areas will be assessed at 1 month, 3 months, and 6 months post-MKTP.
To participate, or for more information, please contact the
Henry Ford Dermatology Research Office at: 313-916-6964. |
Clinical Trial in Canada
Efficacy of Red Light Treatment for Vitiligo
Principal Investigator: Harvey Lui, MD FRCPC
Co - Investigator

Mohammed I AlJasser, MD FRCPC
Study Location:
The Skin Care Center, Vancouver General Hospital
Vancouver, British Columbia, Canada, V5Z 4E8
Prospective single-blind randomized clinical trial to assess the
efficacy of red light treatment in vitiligo. Visible red light has been
shown to stimulate melanocyte migration and proliferation resulting in repigmentation of vitiligo.
Eligibility Requirements:
- Must be a resident of Canada
- Must have localized or generalized vitiligo
- Must have a depigmented area 4 sq. inches (25 cm2) or larger,
(excluding face, genitals, hands and feet) with no more than 10% repigmentation.
Exclusion Criteria:The following will not be eligible:
- Patients who received treatment for vitiligo within the past 3 weeks.
- Patients known to have a photosensitivity disorder
- Patients with a history of previous skin cancer
- Patients with a history of severe medical illness or immunosuppression
- Patients who are pregnant or breast-feeding
The vitiligo patch will be divided into 4 quadrants. One quadrant will
receive low intensity red light, one high intensity red light, and the other
two will be shielded and not receive any treatment. Treatments will be
given twice weekly for 10 weeks, with assessments done every 2 weeks
while receiving treatment, and at 4, 8, and 12 weeks post-treatment.
If you are interested in participating or would like more information:
Contact: Co-Investigator: Mohammed I AlJasser, MD, FRCPC
Phone: 1-778-859-5522 or Email: [email protected] |
UVA1 Phototherapy Study for Vitiligo
Principal Investigator: Harvey Lui, MD FRCPC
Study Location:
The Skin Care Center, Vancouver General Hospital
Vancouver, British Columbia, Canada, V5Z 4E8
Prospective single-blind randomized clinical trial to assess the efficacy of UVA1 phototherapy in vitiligo. UVA1 phototherapy has been shown to be useful for a variety of skin diseases due to its anti-inflammatory effect and deeper penetration. However, there are only a few studies published on the efficacy of UVA1 in vitiligo.
Eligibility Requirements:
- Must be older than 18 years of age
- Must be a resident of Canada
- Must have localized or generalized vitiligo
- Must have a depigmented area 4 sq. inches (25 cm2) or larger,
(excluding face, genitals, hands and feet) with no more than 10% repigmentation.
Exclusion Criteria:The following will not be eligible:
- Patients who received treatment for vitiligo within the past 3 weeks.
- Patients known to have a photosensitivity disorder
- Patients with a history of previous skin cancer
- Patients with a history of severe medical illness or immunosuppression
- Patients who are pregnant or breast-feeding
Treatments will be given Monday to Friday for 8 weeks with a total of 40 treatments. Assessments will be done every 2 weeks while receiving treatment, and at 4, 8, and 12 weeks post-treatment.
If you are interested in participating or would like more information:
Contact: Co-Investigator: Mohammed I AlJasser, MD, FRCPC
Phone: 1-778-859-5522 or Email: [email protected] |
Editors Note: UVA1 is a relatively new form of phototherapy that has been found to be effective in treating several skin disorders, including atopic dermatitis, localized scleroderma, systemic sclerosis, cutaneous T-cell lymphoma, lupus erythematosus, and others. Side effects (burning, hyperpigmentation, redness, itch, etc.) appear less frequently compared to conventional phototherapy, and none serious that can be traced directly to UVA1 treatment, though long-term studies are not yet available. Instances of UV-induced burning were actually less than with broadband UVB or PUVA treatments. UVA1 is reported to induce immediate tanning and pigment darkening. Responses from UVA1 treatments continue even months after the treatment is completed therefore generally requiring fewer overall treatments. UVA1 units consist of metal halide lamps equipped with special optical filters, which emit longer, more penetrating wavelengths than the fluorescent UVA tubes used with PUVA. Both smaller units to treat localized areas and larger cabinets to treat the whole body are available. Use of UVA1 light units are limited in the U.S., due most likely to the high cost of the equipment, which can be 2-3 times more expensive than conventional whole-body light units.
Your Opportunity to Move Research Forward!
University of Colorado
Health School of Medicine
International Study to Find Vitiligo Genes

Update!
Additional Patient Volunteers Needed
From the
USA and Canada
Scientists with the International VitGene Consortium project spanning 18 countries are working to understand the biology of vitiligo so that more effective vitiligo treatments can be designed.
Thanks to your involvement, the first two phases of this research project have been extraordinarily successful, discovering many vitiligo genes, and resulting in real breakthroughs in understanding and opening doors to potential new treatments.
The U.S. National Institutes of Health (NIH) has now awarded a large research grant for a third phase of these studies, to discover additional vitiligo genes, deepen understanding, and provide even more new targets for vitiligo treatment. To accomplish this, they need to enroll 3000 additional Caucasian (white) vitiligo patients over the next two years. Participants must be from the USA or Canada and not have previously taken part.
Your participation is vitally needed. Please complete the questionnaire below and then email directly to Dr. Richard Spritz at the University of Colorado School of Medicine using the email address provided at the end of the questionnaire.
Your personal information, by law,
will be kept private and will not be sold or disclosed.
Join with us to work for a vitiligo-free future!
|

Earn Funding for VSI 3 Ways When You Shop!
Please keep VSI in mind when you do any of your online shopping
AMAZON SHOPPING
Amazon.com has all kinds of items in addition to books. As long as shopping is done through this link, Amazon.com, or from the Amazon box on our Community Home Page, VSI will earn fees, based on a percentage of the sale*. The more items members buy, the higher the percentage! Our Vitiligo Library and Store, containing books, articles and products for those with vitiligo, is also powered by Amazon.
iGIVE SHOPPING
iGive.com is another program with an online shopping mall with over 700 stores where you can shop and earn VSI a percentage-coupons are often available as well.
Let friends and family know about iGive so they can support VSI, too. You do have to register for iGive. Once you've registered, you can either shop directly through their "mall" on iGive's website or by downloading their new button, which makes it even easier. iGive also has a search function powered by Yahoo at isearchigive.com (or through the button) that earns VSI a penny per search. The power of numbers makes this also an effective way to support VSI.
GOODSEARCH SHOPPING AND DINING!
GoodSearch and Goodshop are similar to iGive; click “get started” and designate Vitiligo Support International as your cause - you can also register, or sign in through Facebook, to keep track of your donations (registration is not required). Goodshop has some different stores (like Target), though, and also offers coupons and often a higher percentage to VSI. Goodsearch also offers an optional toolbar. Through GoodDining, participating restaurants automatically donate a percentage of your meal purchases to VSI if you (securely) register your credit cards. If you provide reviews of the restaurants afterwards, we earn even more!
* Vitiligo Support International Inc., (VSI) is a participant in the Amazon Services LLC Associates Program, an affiliate advertising program designed to provide a means for sites to earn advertising fees by advertising and linking to Amazon.com. |
|




 Now Visit VSI
Now Visit VSI  And Twitter
And Twitter










 Research has shown that NB-UVB light does not penetrate the eyelid. As long as no psoralen is being used (as with PUVA), the patient is old enough to understand they must keep their eyes closed at all times, and has an annual eye exam, it should be safe to expose the eyelid to NB-UVB light.
Research has shown that NB-UVB light does not penetrate the eyelid. As long as no psoralen is being used (as with PUVA), the patient is old enough to understand they must keep their eyes closed at all times, and has an annual eye exam, it should be safe to expose the eyelid to NB-UVB light. Unfortunately, this is not true. There are various skin conditions that can cause “hypo-pigmentation,” or loss of pigment. One of the more common, known as tinea versicolor, is caused by a yeast-type fungus, and as such, is treated with antifungal medications. Because (anti) dandruff shampoos contain antifungal ingredients, many times this type of “over-the-counter” product will clear up a condition like tinea versicolor. Vitiligo is not a skin fungus, and cannot be treated with antifungal medications.
Unfortunately, this is not true. There are various skin conditions that can cause “hypo-pigmentation,” or loss of pigment. One of the more common, known as tinea versicolor, is caused by a yeast-type fungus, and as such, is treated with antifungal medications. Because (anti) dandruff shampoos contain antifungal ingredients, many times this type of “over-the-counter” product will clear up a condition like tinea versicolor. Vitiligo is not a skin fungus, and cannot be treated with antifungal medications.


